Please provide values below to convert Atomic mass unit [u] to gram [g], or vice versa.

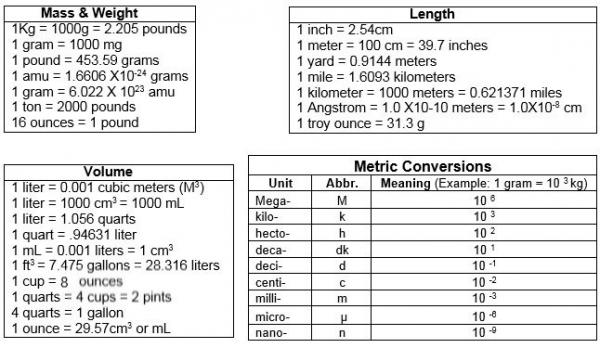
Atomic Mass Unit to Gram Conversion Table

Experiments have shown that 1 amu = 1.66 × 10 −24 g. Mass spectrometric experiments give a value of 0.167842 for the ratio of the mass of 2 H to the mass of 12 C, so the absolute mass of 2 H is mass of 2H mass of 12C × mass of 12C = 0.167842 × 12amu = 2.104104 amu The masses of the other elements are determined in a similar way. Numerical value: 1.660 539 066 60 x 10-27 kg: Standard uncertainty: 0.000 000 000 50 x 10-27 kg: Relative standard uncertainty: 3.0 x 10-10: Concise form 1.660 539 066 60(50) x 10-27 kg: Click here for correlation coefficient of this constant with other constants. The original standard of atomic weight, established in the 19th century, was hydrogen, with a value of 1. From about 1900 until 1961, oxygen was used as the reference standard, with an assigned value of 16. The unit of atomic mass was thereby defined as 1 / 16 the mass of an oxygen atom. In 1929 it was discovered that natural oxygen contains. An atomic mass unit is the same thing as grams per mole (1 amu = 1 g/mol). It is also the same thing as a dalton (1 amu = 1 Da). So if you don't know the amu for one of your elements, you can search for this particular isotope online to find the amu and natural abundance specific to that particular isotope.
| Atomic Mass Unit [u] | Gram [g] |
|---|---|
| 0.01 u | 1.6605402E-26 g |
| 0.1 u | 1.6605402E-25 g |
| 1 u | 1.6605402E-24 g |
| 2 u | 3.3210804E-24 g |
| 3 u | 4.9816206E-24 g |
| 5 u | 8.3027009999999E-24 g |
| 10 u | 1.6605402E-23 g |
| 20 u | 3.3210804E-23 g |
| 50 u | 8.3027009999999E-23 g |
| 100 u | 1.6605402E-22 g |
| 1000 u | 1.6605402E-21 g |
How to Convert Atomic Mass Unit to Gram
1 u = 1.6605402E-24 g
1 g = 6.0221366516752E+23 u
Example: convert 15 u to g:
15 u = 15 × 1.6605402E-24 g = 2.4908103E-23 g
Popular Weight And Mass Unit Conversions
Convert Atomic Mass Unit to Other Weight and Mass Units
Why is the atomic mass unit (amu), rather than the gram, used to express atomic mass?
1 Answer
Because atoms are ridiculously small.
And the
which is immeasurably small. We don't care for masses that small because we physically can't see or measure it. Instead, we care for masses we can touch, like
And that involves:
Amu Chemistry
You can clearly tell that this number of atoms is impossible to count. And so Avogadro's number,
Value Of 1 Amu In Kg
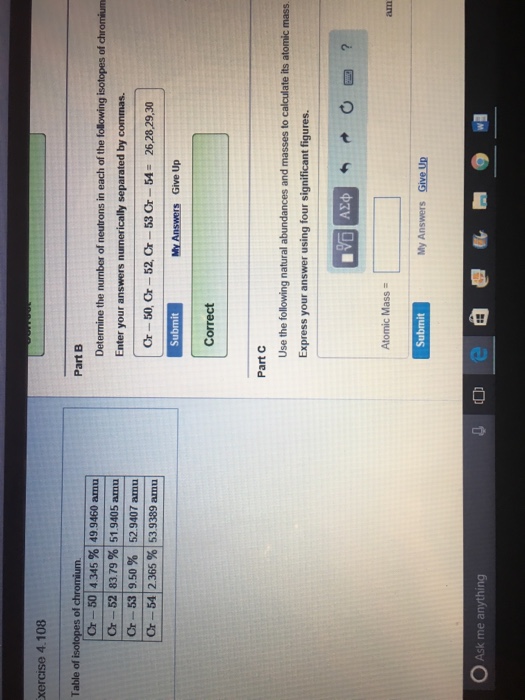
Value Of 1 Amu In Kg
And as you can see, these numbers look much nicer and more physically useful.
How To Find Amu
Related questions
